Updated January 2024
Appendix 1.9 – CCO Staging Guidelines
Appendix 1.9 Reporting of Cancer Stage at Diagnosis by Ontario Regional Cancer Centres
Introduction
The appropriate management of a patient with cancer is not possible without knowledge of the extent or stage of the cancer at diagnosis. Clinicians stage all cancer patients as part of a routine assessment and treatment plan. The American Joint Committee on Cancer Tumor Nodes Metastases (AJCC TNM) staging classification is the accepted staging standard in North America. AJCC TNM8 was adopted in Canada effective with patients diagnosed on January 1, 2018. Prior to 2018, the Collaborative Stage Data Collection System (CS), based on AJCC TNM6 and AJCC TNM7, was in use. NB: Data collected under the CS system continues to be available for research purposes upon request.
Stage information constitutes one of the most important prognostic factors for cancer treatment. Reliable stage information also supports healthcare providers, administrators, researchers and decision-makers in their planning, evaluation, and quality improvement activities meant to enhance the patient experience and to advance patient outcomes.
Ontario Health (Cancer Care Ontario) promotes the use of stage data in the development and reporting of cancer system indicators to facilitate surveillance of prognostically similar groups of cancer; to assess treatment patterns and guideline concordance; to inform decision making processes; and to support stage data quality improvement efforts.
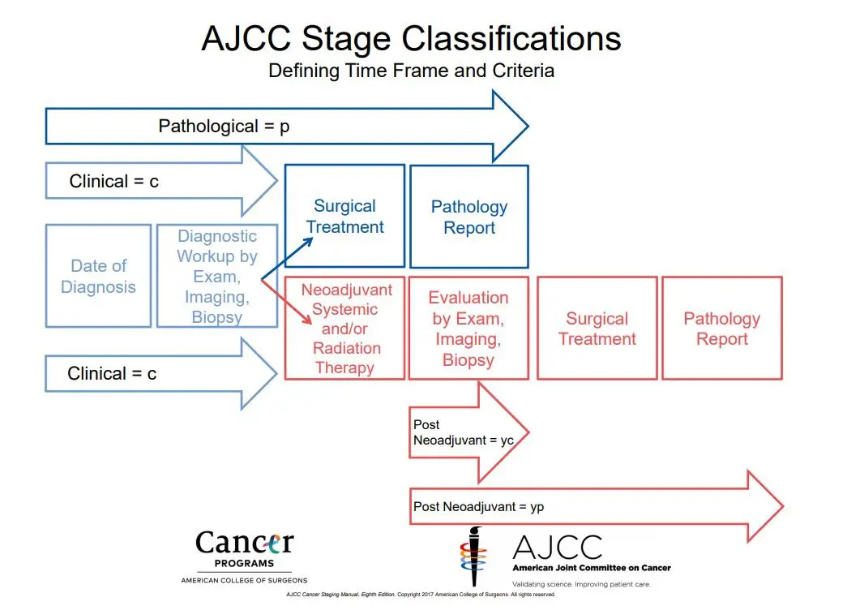
Stage at Diagnosis is defined as the classification of patients with cancer into prognostically similar groups according to the extent of disease at the time of initial diagnosis. All patients undergoing diagnostic work-up for cancer are assigned a clinical stage (c) to develop a treatment plan. If the patient has definitive surgical treatment, the managing physician then assigns a pathological stage (p). If the patient receives neoadjuvant, or pre-surgical, systemic treatment or radiation therapy, the managing physician assigns a post therapy stage (yp) to indicate the response to treatment. Only two of these three stage groups are reported for any given case. e.g., clinical + pathological, clinical + post therapy.
Each treating facility is responsible for ensuring processes are in place for accurate clinical documentation and collection of TNM stage data.
NB: yc (Post-neoadjuvant Clinical) staging is not currently mandated for use in Canada and is not reported to Ontario Health (CCO). However, yp (Post Therapy) is mandated by both when appropriate. To complete yp staging, the results of the neoadjuvant therapy must be considered (essentially using the yc information).
Stage Data Submission Process
AJCC TNM staging is reported centrally by Regional Cancer Centres in Ontario via Ontario Health’s (Cancer Care Ontario) Data Book. This appendix provides an update on that reporting process.
The original policy was…Ontario Health’s (Cancer Care Ontario) “Guidelines for Staging Patients with Cancer” …
https://ext.cancercare.on.ca/ext/databook/db2324/assets/docs/CCOCancerPatientStagingGuidelines.pdf
More recent Quality Based Procedures (QBP) Annual Funding Agreements, also outline the responsibilities of regional cancer centres as defined.
Excerpt taken from 2023 - 2024 QBP Funding Agreement:
2. With respect to cancer staging, as a provider of cancer services, the Recipient will:
2.1 Ensure that all eligible cancer patients are staged according to pan-Canadian endorsed standards of the American Joint Committee on Cancer (AJCC) most current edition Tumor Node Metastasis (TNM) staging authorized for use in Canada (i.e. AJCC 8th Edition for patients diagnosed in the calendar year 2018 onwards and AJCC TNM version 9 for patients diagnosed with cancer of the cervix in the calendar year 2021 onwards, and cancer of the anus and appendix, 2023 onward). Regional Cancer Centres (RCCs) are also required to electronically submit TNM staging for all eligible cancer patients, as outlined in the Data Book reporting requirements, remembering that submission of stage for breast, colorectal, lung, prostate, and cervix cancer is not mandatory.
2.2 Continue to provide remote access to the Recipient’s electronic health record, for OH staging analysts, as outlined in a separate OH and Recipient agreement entitled “Access to Cancer Patient Health Records for Collection of Cancer Staging Information” (2008-2009; 2010; 2011).
2.3 Appoint or reconfirm a Stage Physician Lead and a Health Records Stage Lead to serve as a single point of contact with OH on stage capture issues. The attached template (“Cancer QBP Agreement Recipient Contact Profile FY23-24”) must be completed and submitted to OH as detailed in Schedule “C”.
For a full list of Regional Cancer Centres and affiliated reporting hospitals, see Data Book Appendix 1.34
https://ext.cancercare.on.ca/ext/databook/db2324/Appendix/Appendix_1.34_-_Reporting_Facilities.htm
Ontario Health (Cancer Care Ontario) operates the Ontario Cancer Registry (OCR) with a mandate to collect stage data at a population level, in compliance with national standards set by the Canadian Council of Cancer Registries (CCCR), to populate the Canadian Cancer Registry (CCR), operated by Statistics Canada. The stage data set required by the national registry exceeds that which is currently submitted through Data Book. Consequently, the OCR retains the primary responsibility for staging those cancer sites mandated by the Canadian Partnership Against Canada (CPAC)…Breast, Colorectal, Lung and Prostate. NB: Cervix is also staged by the OCR to support the provincial screening program. Therefore, submission of stage data by Regional Cancer Centres via Data Book for Breast, Cervix, Colorectal, Lung and Prostate cancers is optional, although it is strongly advised that each centre collect this data for their own research purposes.
Submission of AJCC TNM stage data by Regional Cancer Centres is mandatory for all other primary cancer sites when there is a corresponding AJCC TNM staging schema and when the case is analytic for that centre.
While Ontario Health (CCO) encourages all facilities to collect stage at diagnosis, on as many cases as possible, the following stageable cases are excluded: non-reportable cases and non-analytic cases.
For the definition of an Analytic Case see Appendix 1.16 – Analytic Case Flag.
https://ext.cancercare.on.ca/ext/databook/db2324/Appendix/Appendix_1.16_-_Analytic_Case_Flag_.htm
Stage at diagnosis is to be recorded for all invasive and in-situ cancers with an AJCC stage schema and submitted to Ontario Health (Cancer Care Ontario) monthly via the Data Book reporting process, with exceptions noted elsewhere in this document. Stage should be submitted within 6 months from the date of diagnosis (Regional Programs Performance Indicator) and no later than 10 months (Ontario Cancer Registry).
Ontario Health (Cancer Care Ontario) through the Ontario Cancer Registry will provide performance metrics to RCCs on the completeness of their stage data (Standard: 90% completeness rate), will notify RCCs of relevant stage training opportunities, and will attempt to address all cancer staging and coding questions posed in the ocrquestions mailbox. OH-CCO, ocrquestions OH-CCO_ocrquestions@ontariohealth.ca
All RCCs should have a policy for the identification, collection and processing of cancer stage related data in their organization. Each centre will ensure compliance with that policy and enact processes to guarantee that the stage information is of high quality. Specific strategies to support quality may include regular review of incomplete cases, monitoring frequency of unknown stage, conducting restaging audits, and validation studies of stage by HIM staff, Cancer Registrars and/or Clinician experts.
AJCC TNM Staging Rules
To verify the eligibility of a case for AJCC TNM staging, based on the topography and histology codes, refer directly to the Chapter in the AJCC TNM8 Staging Manual and/or the AJCC TNM version 9 publication, depending on the diagnosis year.
CANCER PROGRAMS
AJCC Version 9 Cancer Staging System
https://www.facs.org/quality-programs/cancer-programs/american-joint-committee-on-cancer/version-9/
AJCC Protocol for Cancer Staging Documentation, Sites Now Staged Under Version 9
|
Primary Site |
AJCC TNM Version 9 Effective Date |
|
NET Appendix |
January 1, 2024 |
|
NET Colon and Rectum |
January 1, 2024 |
|
NET Duodenum and Ampulla Vater |
January 1, 2024 |
|
NET Jejunum and Ileum |
January 1, 2024 |
|
NET Pancreas |
January 1, 2024 |
|
NET Stomach |
January 1, 2024 |
|
Vulva |
January 1, 2024 |
|
Appendix |
January 1, 2023 |
|
Anus |
January 1, 2023 |
|
Brain & Spinal Cord |
January 1, 2023 |
AMERICAN JOINT COMMITTEE ON CANCER
Cancer Staging Systems
Cancer Staging Education

CANCER PROGRAMS
Physician Education
Cancer Staging System Webinars
AJCC Cancer Staging Manual in the Literature
Cancer Registrar Education
Sample of videos available:
AJCC TNM8 Overview
Head and Neck Staging
Melanoma Staging
Blank Vs X Definitions and Data Interpretation for AJCC Staging
Ambiguous Terminology

https://www.facs.org/media/2zvlrdmi/ajcc_staging_rules_8th_ed.pdf